Home \ Knowledge Hub \ Focus on \ Focus on… Using Models to Help Control Infectious Disease


10 May 2021
Focus on… Using Models to Help Control Infectious Disease
SHARE

EMMA TAYLOR
PhD Candidate, University of Surrey, School of Veterinary Medicine
COVID-19 is not the first and won’t be the last zoonosis
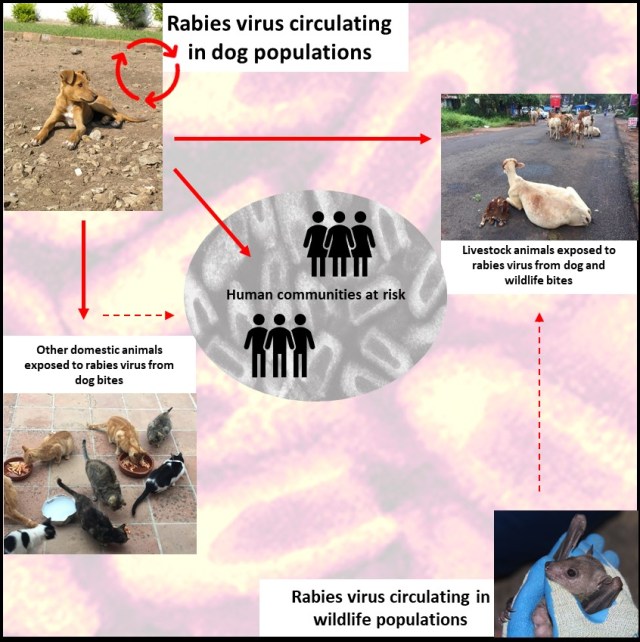
Tackling the human health consequences of diseases that originate in animals is not a new problem. Long before the global COVID-19 crisis, efforts were being made globally to fight zoonotic diseases. One of the oldest known zoonoses is rabies, which has a uniquely high mortality rate killing over 60,000 people every year. Although also caused by a virus, there are important differences between rabies and COVID-19: we know that dogs are the primary source of human rabies and therefore dog vaccination will eventually stop human cases (Fig 1.). It should therefore be possible to eliminate dog mediated rabies, and there is a global effort to do so by 2030, supported by WHO, FAO and OIE. The COVID-19 pandemic has the potential to derail these efforts in some areas, through diversion of resource or restrictions. However, there are some reasons to remain optimistic. One of these is that public awareness of the complexity of disease control methods has increased, which gives us the opportunity to build public trust in how, where and when we use models to help control diseases.
“All models are wrong, but some are useful”
This expression, coined by the statistician George Box, describes the complexities associated with modelling the approximate representation of the truth that is provided by imperfect data. Models are useful to infer things we don’t yet know about from the things we think we do (so called ‘parameters’).
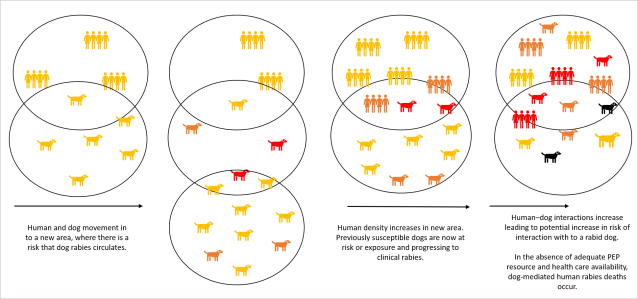
The mechanisms involved in dog rabies transmission, i.e. from infected dog to susceptible human, are well understood, however transmission interactions throughout the two populations are complex and the large scale dynamics which impact disease spread can be difficult to disentangle without mathematical modelling. For example, if we considered just two dogs living alongside one another, and one of those dogs was infected with rabies, we could relatively easily predict the likelihood of it infecting the other dog without models. However, when there are hundreds of dogs living across interconnected villages, where hundreds of potentially susceptible people are living, it soon becomes complicated (Fig 2.). Here, we can use modelling to work with these large datasets to complete these predictions. Examples of this approach have come into the public eye recently for COVID-19, where governments across the world have relied on predictions from mathematical models to make decisions during the pandemic (Adam, 2020; Poletto et al., 2020).
How do we use models to help rabies control?
One early example of applying mathematical models to rabies control comes from attempts to understand fox rabies in Europe, developed by Anderson and May (1981). This compartmental modelling approach helps to explain the dynamics involved in the spread of rabies virus in a fox population by allocating foxes into one of several virtual groups , and using equations to simulate the rates at which foxes ‘move’ between the compartments based on what was known about the animals, and the virus at the time- a so called ‘susceptible, exposed, infectious, recovered’ (SEIR) model.
These models were considered important in supporting vaccination of foxes, which is the pre-eminent worldwide example of successful control of an infectious disease in a wildlife population. Within the current all-important dog rabies control efforts, mathematical modelling has also contributed to policy development, and provided evidence to inform progress towards the “Zero by 30” strategic plan to end human deaths from dog-mediated rabies by 2030 (Hampson, 2020; Kleczkowski et al., 2019).
The current World Health Organisation Technical Guidance specifically recognises the contribution of modelling in controversial and complex subjects such as the relative benefits of sterilisation of dogs in addition to vaccination, and accurately quantifying the burden of rabies where data are missing (World Health Organization, 2018).






Despite rabies in people being 100% preventable, progress towards control is challenging due to, for example the lack of data available on dog demographics, lack of awareness to the risk, poor resource allocation and lack of consistent surveillance. With the support of my supervisors Dr Dan Horton, and Dr Joaquin Prada at the University of Surrey, UK, and in collaboration with Centre for Disease Control, Atlanta (CDC), and the Global Alliance for Rabies Control (GARC), we are applying a combination of modelling approaches, which capture disease dynamics and the economic impact of rabies control, to create a new tool to improve strategic risk management and resource allocation for rabies programmes.
Establishing new approaches and adapting current strategies
The COVID-19 pandemic has heightened public awareness to the importance of surveillance, vaccination access, and resource allocation. It is likely that COVID-19 will continue to impact efforts towards control and elimination of other diseases, including rabies, it is thus imperative to establish new approaches to rabies control, and adapt current ones. Considerations such as how to deliver dog vaccination while accommodating COVID-19 restrictions and planning for interruptions to current campaigns, become more important as the COVID-19 situation evolves.
References
Adam, D., 2020. Special report: The simulations driving the world’s response to COVID-19. Nature 580, 316–318. https://doi.org/10.1038/d41586-020-01003-6
Hampson, K., 2020. Zero human deaths from dog-mediated rabies by 2030: perspectives from quantitative and mathematical modelling. Gates Open Res 3. https://doi.org/10.12688/gatesopenres.13074.2
Kleczkowski, A., Hoyle, A., McMenemy, P., 2019. One model to rule them all? Modelling approaches across OneHealth for human, animal and plant epidemics. Philosophical Transactions of the Royal Society B: Biological Sciences 374, 20180255. https://doi.org/10.1098/rstb.2018.0255
Poletto, C., Scarpino, S.V., Volz, E.M., 2020. Applications of predictive modelling early in the COVID-19 epidemic. The Lancet Digital Health 2, e498–e499. https://doi.org/10.1016/S2589-7500(20)30196-5
World Health Organization (Ed.), 2018. WHO Expert Consultation on Rabies: third report, WHO technical report series. World Health Organization, Geneva, Switzerland.
EMMA TAYLOR
PhD Candidate, University of Surrey, School of Veterinary Medicine
The views expressed in this article are those of the author(s) and do not necessarily represent those of MSD Animal Health.

