Home \ Knowledge Hub \ Focus on \ Focus On… Avian Influenza – a unique challenge


01 Oct 2017
Focus On… Avian Influenza – a unique challenge
SHARE

PAUL F. MCMULLIN
RCVS Specialist in Poultry Medicine and Production, Poultry Health Services, North Yorkshire
Influenza viruses occur naturally in many wild birds and, periodically, some of these ‘spill over’ into poultry, pigs, and humans. The winter of 2016/17 has seen widespread occurrence of a new strain of highly pathogenic avian influenza in many countries in Eurasia, with spread even to southern Africa. The condition can cause high mortality, poor poultry welfare, and severe economic effects for farmers. However, these effects are dwarfed by the economic effects of control measures which can severely restrict trade, not just in poultry products, but also in key inputs such as breeding stock. Achieving effective animal health regulation for a condition in which there is a large, intercontinentally mobile, wildlife reservoir poses unique challenges.
How it started – ‘Perroncito’s disease’
In February 1878 Prof. Edoardo Perroncito read a report to his colleagues in the Accedemia di Agricoltura di Torino on a remarkable occurrence in the hills and plains of Piedmonte, the north-western corner of Italy. He described a classic epidemic curve affecting the poultry population, with a few cases initially in the autumn of 1877, then large numbers of cases in many towns and villages, and then a reduction. The disease began in a mild form, and, in some villages, remained so, but in others it became so severe that it “effectively emptied the poultry houses”. He reported that “in addition to the common and foreign breeds of hen, chickens of India, ducks and geese were infected in many places”. While he described it as a “typhoid epizootic”, it is believed to have been the first formal report of what we now call highly pathogenic avian influenza (HPAI) (and, probably, low pathogenic avian influenza). Cases in the following years showed clear links with what we would now describe as ‘poor biosecurity practices’, including some long-distance and international spread by poultry trade and because of an outbreak in a poultry fair. There were other causes of high mortality in this period (in particular fowl cholera and what we now call Newcastle disease) so there is some uncertainty as to which outbreaks were due to which infection in the years before and after Perroncito’s report.
Epidemiology – what we know now
There is growing evidence of the role of wild birds in the epidemiology of influenza viruses and recognition that wild birds can be a source of highly pathogenic infection for commercial poultry. The avian viruses that have the potential to cause a pandemic in human population caused a lot of concern between 2004–2007 and between 2015–2017 they were ’back with a bang’.

Since then, HPAI has occurred sporadically, with outbreaks ranging from individual small flocks to widespread occurrence affecting many millions of birds in multiple countries. Although we know that ducks and geese tend to be more resistant to the infection than chickens, over the years, they have been involved in many outbreaks, including the originally described one. Relatively little appears to be known of the epidemiology and effects of influenza viruses in wild birds prior to 2003. Only one clinical outbreak had been identified—resulting in the death of about 1300 terns in South Africa in 1961. That particular sub-type (H5N3) was not identified in any of the poultry outbreaks in the following 40 years. Since 2004 there is growing evidence of the role of wild birds in the epidemiology of influenza viruses. We know that a broad range of influenza types circulate naturally, and usually asymptomatically, in many wild bird populations— particularly birds associated with aquatic habitats. Since the widespread occurrence of H5N1 outbreaks in the period 2004–2007 there has been growing recognition that wild birds can also sometimes be a source for commercial poultry of highly pathogenic forms of the viruses. Much of the public interest at this time focused on the potential for the adaptation of avian virus to spread in the human population and cause a pandemic. While press interest in avian influenza has since waned somewhat, these viruses have not gone away, and, in the period 2015–2017 are certainly ’back with a bang’.
Keeping a watch with growing scrutiny
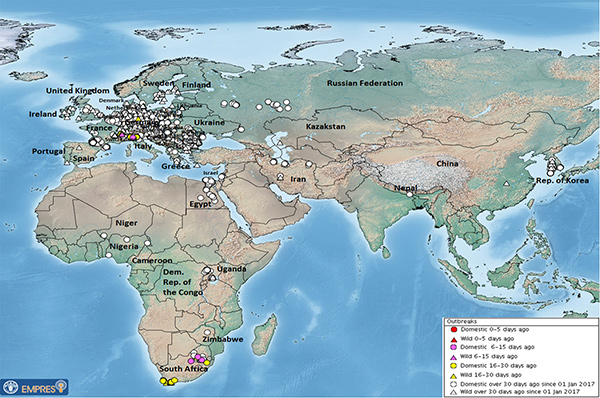
The spread of H5N8, from east Asia, through most of Europe in the winter of 2016/17 and its recent spread to east and southern Africa is an exceptional occurrence.

Modern molecular techniques are greatly increasing our understanding of the complex relationships between the various viruses and changes in virus populations. Now we talk not just about a specific type (e.g., H5N8), but also the clade (in this case 2.3.4.4)—a phylogenetic grouping of all individuals believed to have a common ancestor. The spread of H5N8, from its apparent origin in east Asia, through most of Europe in the winter of 2016/17 and its recent spread to east and southern Africa is a really exceptional occurrence. There is always, of course, the possibility of ‘increased ascertainment’—what most people would express as ‘the more we look, the more we find’. It seems likely, for instance, that publicity with respect to controls required for commercial poultry increased the likelihood of affected very small, isolated, backyard-flock outbreaks being identified. On the other hand, the very dramatic losses seen in affected commercial poultry flocks makes it unlikely that these would be missed, unless there is significant spread of similar low-pathogenic strains in an area (providing some immunity) and/or the presence of significant numbers of the less susceptible domestic species (ducks and geese).
What we need: dosimetry of regulation
Never has it been more important for all of us involved in poultry production to play our part in ensuring that the economic, veterinary, and medical risks of these infections are minimised. Sound regulation and control must be based on a good understanding of the epidemiology. At a recent technical meeting a senior veterinarian involved in avian influenza regulation made the point that the key thing is the correct ‘dosimetry of regulation’, paraphrasing Paracelsus (1493–1541) who said “The right dose differentiates a poison from a remedy”. Excessive, heavy-handed regulation has the potential to produce the opposite effect to that intended, by, for instance, reducing public support for the control measures adopted or reducing cooperation from those who are regulated. It may also hinder the adaptations of controls to the requirements of particular countries or prevent the development and application of new approaches to disease control.
Regulatory framework must catch up with recent advances in our understanding of avian flu. A broad international agreement on control measures will help protect the health of both poultry and wildlife and minimise disruption to trade.
We understand that the code commission of the OIE has included a review of the avian influenza chapter of its Terrestrial Code in its work programme. This will be a good opportunity to ensure that the regulatory framework catches up with recent advances in our understanding of ‘Perroncito’s disease’, and it is hoped that this review will proceed as a matter of urgency. Getting broad international agreement on control measures will help protect the health of both poultry and wildlife while minimising unnecessary disruption to trade.
Perroncito himself would, no doubt, be amazed by many things in our world, not least that we are still quoting from his report of 1878 almost 140 years later!
PAUL F. MCMULLIN
RCVS Specialist in Poultry Medicine and Production, Poultry Health Services, North Yorkshire
The views and opinions expressed in this article are those of the author.

